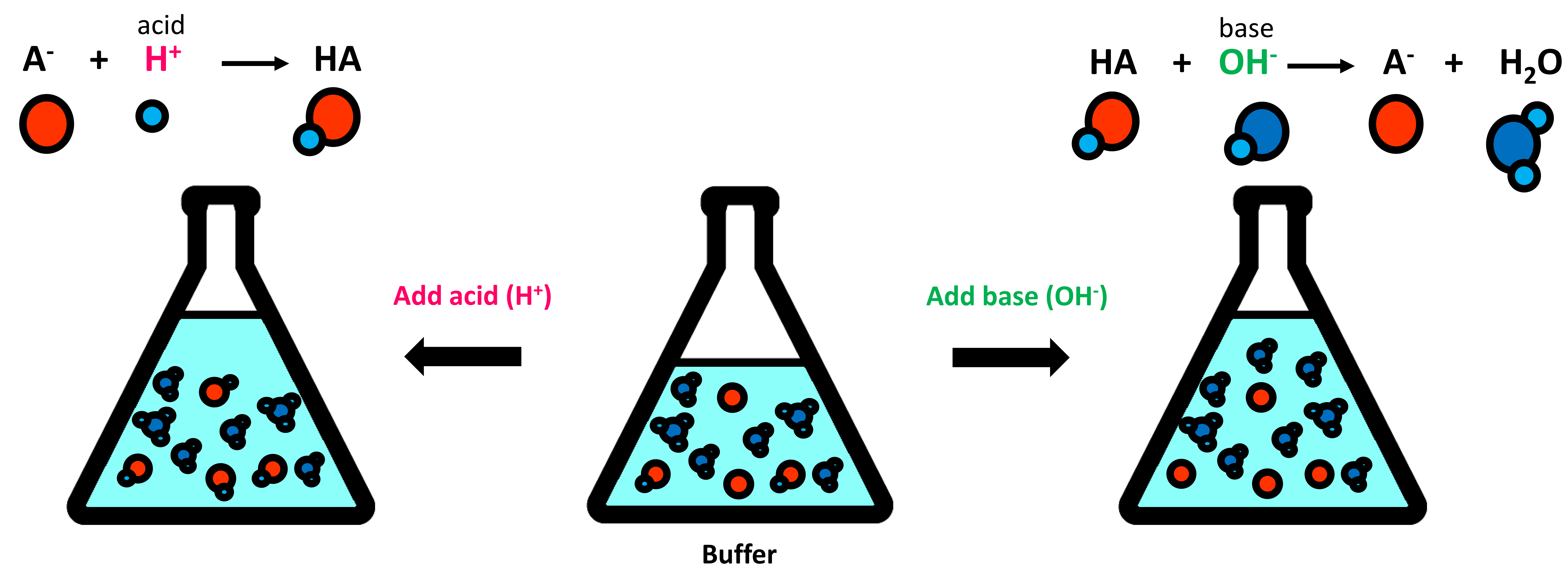
Common Biological Buffers . the use of buffers that mimic biological solutions is a foundation of biochemical studies. They act by neutralizing excess. In this way, a biological. One of the most common. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. biological buffers are organic substances that help regulate the ph level in organisms. choosing the right biological buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. (1) the bicarbonate buffer system and (2) the phosphate buffer. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. there are two classes of buffers which play crucial roles in the human body:
from goldbio.com
a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. One of the most common. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. (1) the bicarbonate buffer system and (2) the phosphate buffer. there are two classes of buffers which play crucial roles in the human body: They act by neutralizing excess. choosing the right biological buffer. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological.
What is a Biological Buffer and How to Choose the Best Buffer for Your
Common Biological Buffers (1) the bicarbonate buffer system and (2) the phosphate buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. choosing the right biological buffer. In this way, a biological. They act by neutralizing excess. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. biological buffers are organic substances that help regulate the ph level in organisms. the use of buffers that mimic biological solutions is a foundation of biochemical studies. there are two classes of buffers which play crucial roles in the human body: One of the most common. (1) the bicarbonate buffer system and (2) the phosphate buffer. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions.
From studylib.net
Good's buffers (biological buffers) Common Biological Buffers the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the use of buffers that mimic biological solutions is a foundation of biochemical studies. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. One of the most common. biological buffers are organic substances. Common Biological Buffers.
From exonxjimh.blob.core.windows.net
Common Buffers Used In Biochemistry at Betty Beckles blog Common Biological Buffers the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological. One of the most common. choosing the right biological buffer. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. there are two classes of buffers which play crucial. Common Biological Buffers.
From pubs.acs.org
Interactions of Common Biological Buffers with Iron Oxide Nanoparticles Common Biological Buffers They act by neutralizing excess. choosing the right biological buffer. One of the most common. In this way, a biological. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Choose a buffer based on. Common Biological Buffers.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every Common Biological Buffers They act by neutralizing excess. the use of buffers that mimic biological solutions is a foundation of biochemical studies. there are two classes of buffers which play crucial roles in the human body: a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. choosing the right biological buffer. the buffer. Common Biological Buffers.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Common Biological Buffers the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the use of buffers that mimic biological solutions is a foundation of biochemical studies. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. (1) the bicarbonate buffer system and (2) the phosphate buffer.. Common Biological Buffers.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog Common Biological Buffers a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. (1) the bicarbonate buffer system and (2) the phosphate buffer. biological buffers are organic substances that help regulate the ph level in organisms. choosing the right biological buffer. One of the most common. They act by neutralizing excess. the buffer systems. Common Biological Buffers.
From www.whdsbio.cn
Common uses and precautions for MES bufferblood collection,Biological Common Biological Buffers a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. there are two classes of buffers which play crucial roles in the human body: choosing the right biological buffer. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma. Common Biological Buffers.
From animalia-life.club
Phosphate Buffer System Equation Common Biological Buffers In this way, a biological. there are two classes of buffers which play crucial roles in the human body: choosing the right biological buffer. One of the most common. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Choose a buffer based on your ph requirements as well as. Common Biological Buffers.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Common Biological Buffers (1) the bicarbonate buffer system and (2) the phosphate buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the use of buffers that mimic biological solutions is a foundation of biochemical studies. biological buffers are organic substances that help regulate the ph level in organisms. a biological. Common Biological Buffers.
From studylib.net
Buffers for Biological Systems Common Biological Buffers biological buffers are organic substances that help regulate the ph level in organisms. the use of buffers that mimic biological solutions is a foundation of biochemical studies. (1) the bicarbonate buffer system and (2) the phosphate buffer. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. One of the most common.. Common Biological Buffers.
From rowangroboone.blogspot.com
Certain Proteins Act as Buffers in the Blood RowangroBoone Common Biological Buffers In this way, a biological. One of the most common. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. there are two classes of buffers which play crucial roles in the human body: (1) the bicarbonate buffer system and (2) the phosphate buffer. They act by neutralizing excess. the buffer systems. Common Biological Buffers.
From www.biobasic.com
Common Buffers Biological Buffers Biochemicals Products Common Biological Buffers the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. there are two classes of buffers which play crucial roles in the human body: In this way, a biological. . Common Biological Buffers.
From www.youtube.com
Buffer action in the blood YouTube Common Biological Buffers One of the most common. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. the use of buffers that mimic biological solutions is a foundation of biochemical studies. In this way, a biological. They act by neutralizing excess. choosing the right biological buffer. there are two classes of buffers. Common Biological Buffers.
From www.crawfordscientific.com
Buffer choice for HPLC separations Common Biological Buffers One of the most common. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They act by neutralizing excess. there are two classes of buffers which play crucial roles in the human body: biological buffers are organic substances that help regulate the ph level in organisms. the use. Common Biological Buffers.
From docslib.org
Biological Buffers Reference Chart DocsLib Common Biological Buffers biological buffers are organic substances that help regulate the ph level in organisms. the use of buffers that mimic biological solutions is a foundation of biochemical studies. (1) the bicarbonate buffer system and (2) the phosphate buffer. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. the buffer systems. Common Biological Buffers.
From goldbio.com
How to Choose the Right Biological Buffer for My Experiment GoldBio Common Biological Buffers Choose a buffer based on your ph requirements as well as the pka, a measure of acid. choosing the right biological buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. biological buffers are organic substances that help regulate the ph level in organisms. a biological buffer is. Common Biological Buffers.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Common Biological Buffers choosing the right biological buffer. One of the most common. the use of buffers that mimic biological solutions is a foundation of biochemical studies. In this way, a biological. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Common Biological Buffers.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Common Biological Buffers the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. biological buffers are organic substances that help regulate the ph level in organisms. They act by neutralizing excess. One of the most common. (1) the bicarbonate buffer system and (2) the phosphate buffer. choosing the right biological buffer. Choose a. Common Biological Buffers.